Excellence in Steroidal API solutions: Precision, Purity, Performance
As a leading company in complex steroidal Active Pharmaceutical Ingredients (APIs), we are driven by a vision to be at the forefront of delivering high-quality APIs that improve health and well-being worldwide.
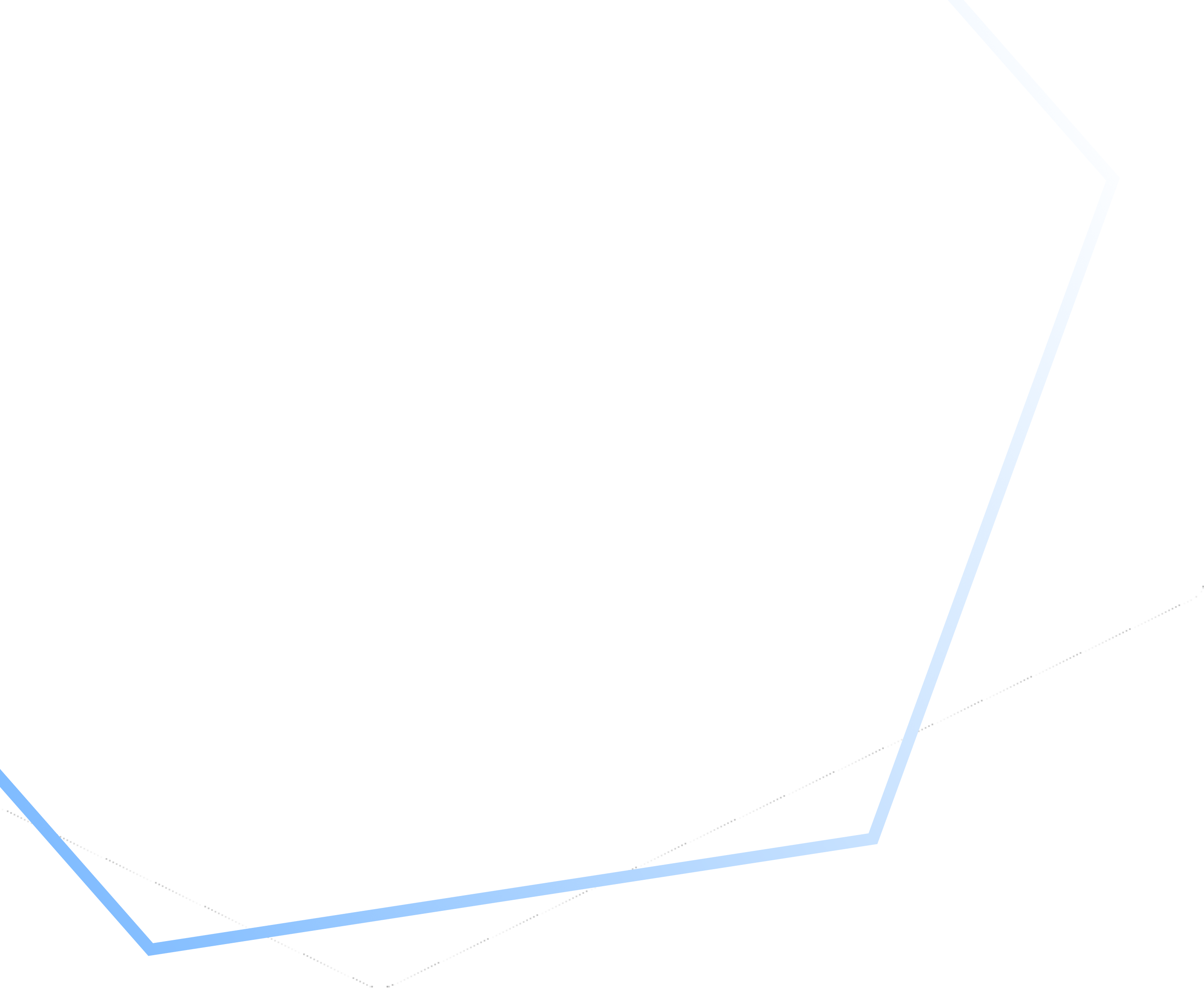
250+
Clients
80+
Countries Worldwide
78+
API Products
20+
Years
20+
Certifications






.png)
.png)








